To conduct a Neutron Activation Analysis experiment, the sample is exposed to neutrons in a nuclear reactor, causing a portion of the atoms to undergo neutron capture: this produces high energy compound nuclei which rapidly transform to radioactive forms of the original chemical element(s). As the short-lived radioactive isotopes undergo decay to reach stable ground state configurations, the sample is placed on a high purity germanium detector which records the intensities and energies of the gamma rays that are emitted. Because a given radioactive isotope always emits gamma rays at certain specific energies and intensities, the radioisotopes present, and hence the parent chemical element(s) present in the sample can be determined quantitatively. This “Short-Lived Neutron Activation Analysis” (SLNAA) technique can be used to quantify dozens of chemical elements including metals, non-metals and metalloids at the parts per million level.
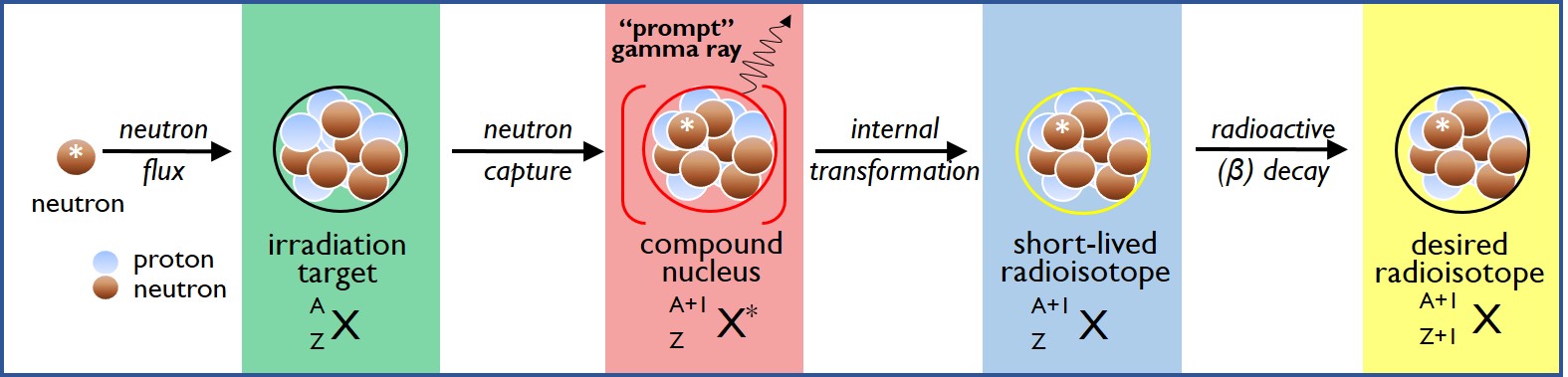
Variations on the SLNAA technique include Prompt Gamma Neutron Activation Analysis, which uses the “prompt” gamma rays emitted during neutron capture, rather than the gamma rays that are emitted as the resulting radioisotope undergoes decay, in order to determine elemental composition; PGNAA is particularly useful for quantifying boron, cadmium, and certain lanthanoids (Eu, Gd, Sm).
A third permutation is Delayed Neutron Counting NAA, which is used primarily for the quantification of uranium. Unlike of the lighter elements in the Periodic Table, when uranium absorbs a neutron, the neutron capture event is followed immediately by nuclear fission. At the uranium atom splits, it ejects an average of 2.8 neutrons per fission; by quantifying (counting) the ejected neutrons, the amount of uranium initially present in the sample can be determined.
NAA is a highly sensitive analytical technique, particularly with the high neutron fluxes available at the McMaster Nuclear Reactor, and can be applied to any element that possesses a suitable activation product (radioisotope). With appropriate experimental parameters, excellent sensitivity is possible for some 70 elements; depending on sample composition, multi-element analysis at the ppm level for up to 30 elements can be accomplished with a single 1 gram sample. Typical analytical applications of SLNAA and PGNAA include determining major and trace elements in a wide variety of materials, including rocks and other geological samples, as well as ceramics, oils, plastics, metals, water, biological and botanical materials. DNC NAA is most commonly used for determining uranium content in soil samples for the uranium mining industry. A significant advantage of NAA over other analytical techniques such as ICP-MS is the simplicity of sample treatment before analysis: in most cases, the only requirement is that the sample be reduced to a size suitable for encapsulation (< 2 mL). Activation analysis is also non-destructive, and can therefore be used for expensive or irreplaceable samples such as archaeological artifacts.
