With a core of enriched uranium fuel assemblies in place, a nuclear reactor can be “started up” using a neutron source: a radioactive substance that emits enough neutrons to jump-start the nuclear chain reaction described above. One commonly used neutron source is californium-252, which spontaneously undergoes both alpha decay and nuclear fission, generating neutrons. Another option is to use a mixture of the chemical element beryllium and a radioactive isotope of antimony (antimony-124 or Sb-124): in this case, the high energy gamma ray given off by the Sb-124 as it undergoes radioactive decay interacts with the nearby beryllium atoms, causing them to eject neutrons. An antimony-124/beryllium neutron source encased in aluminum (so as to avoid contamination of the pool water) is used at MNR to initiate a nuclear chain reaction in the reactor core after protracted shutdowns.
Along with a means of instigating nuclear fission in the reactor core, it is important to be able to control the rate at which the uranium atoms are undergoing fission. If the nuclear chain reaction is allowed to propagate unchecked, the rate of fission will increase rapidly as more and more neutrons are produced, resulting in a massive and nearly instantaneous release of energy and heat. In contrast, in a nuclear reactor, the rate at which the nuclear fuel undergoes fission is rigorously controlled using “control rods”: cylinders of neutron absorbing material that are inserted into the reactor core to absorb a portion of the neutrons released by the fission of the uranium-235 fuel. To initiate a chain reaction when a reactor is being started up, the control rods are partially withdrawn so that they absorb fewer neutrons; once the reactor is critical (i.e. a chain reaction is occurring), the control rods are inserted slightly further into the core in order to slow the rate of fission to the required value and maintain it at that level.
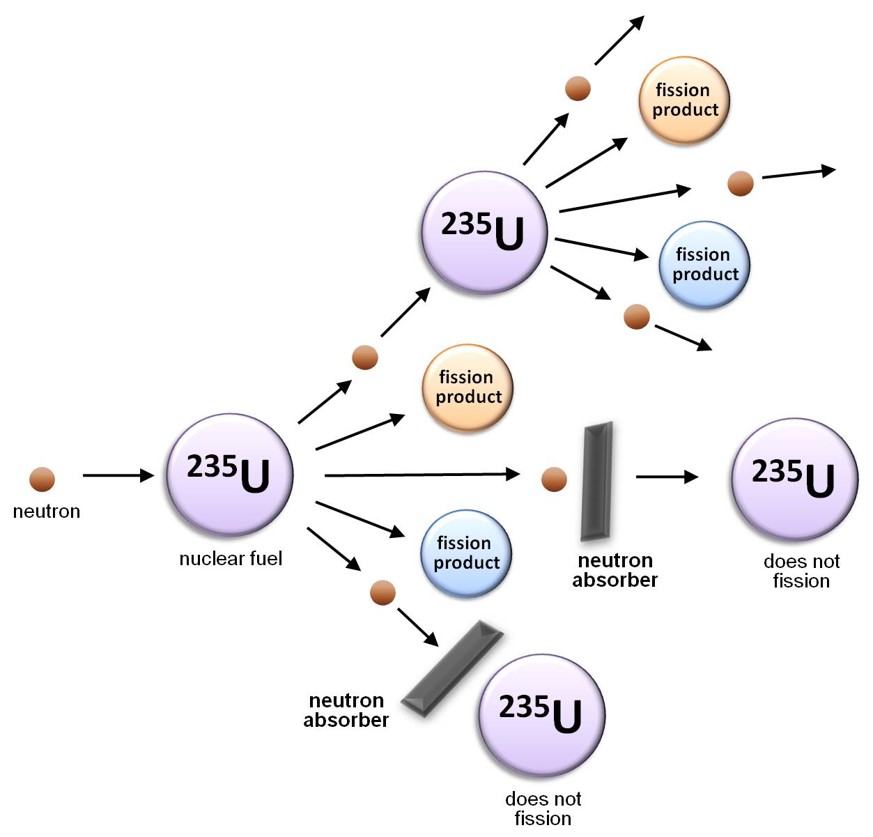
Control rods must be fabricated from a material that readily absorbs or “captures” neutrons, but is not prone to nuclear fission as a consequence. Materials appropriate for this application include boron carbide and the heavy metal cadmium. MNR uses control rods composed of an alloy of silver, indium and cadmium because each of these three metals is able to capture neutrons of a different kinetic energy than the other two, making the alloy highly efficient at regulating the neutron population or “flux” within the reactor core, and thereby controlling the rate of fission.

The other significant component in controlling the rate of fission in a nuclear reactor is the “moderator”. When uranium-235 undergoes fission, the atom flies apart, ejecting fission products and neutrons that are travelling at ~14,000 km/s (50 million km/h). These so-called fast neutrons can cause induced nuclear fission in other uranium-235 atoms, but the probability of this occurring is relatively low: neutron capture and subsequent fission is approximately a thousand times more likely to occur for slower-moving or “thermal” neutrons. The role of the neutron moderator is to decrease or moderate the speed (kinetic energy) of the fast neutrons and convert them to slow moving thermal neutrons to enhance nuclear fission. Of course, the term “slow” is relative: thermal neutrons are still travelling at approximately 2,200 m/s, or 7,920 km/h!
A material suitable for use as a moderator must be able to slow down neutrons without absorbing or “capturing” them. Commonly used neutron moderators include deionized (purified) water, “heavy water” (in which the hydrogen atoms of the water molecules are replaced by the heavier isotope deuterium) and graphite, a high-density form of carbon. Heavy water has the advantage of absorbing very few neutrons, allowing the nuclear chain reaction to progress efficiently, but is far more expensive than regular “light” water. Like the majority of nuclear reactors operational today, McMaster Nuclear Reactor utilizes stringently purified light water as its moderator, as the ready availability of light water is more than sufficient compensation for the decreased fission efficiency that results from its neutron absorptivity. In many nuclear reactors, including MNR, the moderator also doubles as a reactor coolant.
