The Reactor
What is “Nuclear Fission”?
Nuclear fission is a naturally occurring phenomenon during which an atomic nucleus is divided into two or more parts. This is often accompanied by the emission of neutrons, charged fragments such as alpha particles, and energy in the form of gamma or X-rays. Nuclear fission only occurs in the heaviest elements of the periodic table: principally, the actinoid series, but more generally, those with atomic weights greater than 232 atomic mass units. Examples of materials in nature that spontaneously undergo nuclear fission are uranium-235 (an atomic nucleus containing 92 protons and 143 neutrons) and thorium-232 (90 protons and 142 neutrons). Despite being a spontaneous process, fission of these nuclei occurs very slowly in nature: the fission half-life of uranium-235 is 1.2 x 1017 years, which corresponds to one fission per gram per hour.
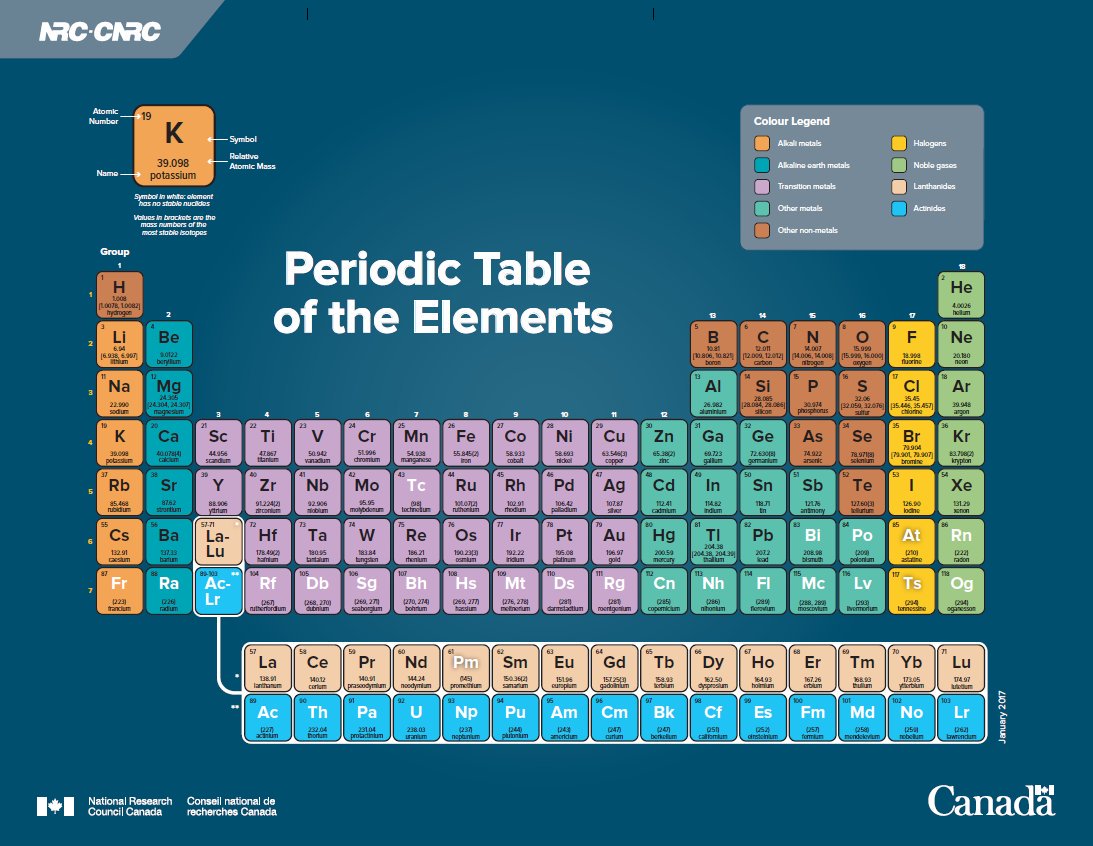
Induced Nuclear Fission
In addition to nuclei that fission spontaneously, many other atomic nuclei can be induced to undergo nuclear fission by bombarding them with sub-atomic particles. For example, a slow-moving or “thermal” neutron can be absorbed into the nucleus of an atom, rendering it unstable and causing it to fission instantly. Nuclei that are susceptible to this “induced nuclear fission” include plutonium-239 and uranium-233. Neutron bombardment can also be used to increase the rate of nuclear fission in nuclei that undergo this process spontaneously, such as uranium-235 (see Figure).
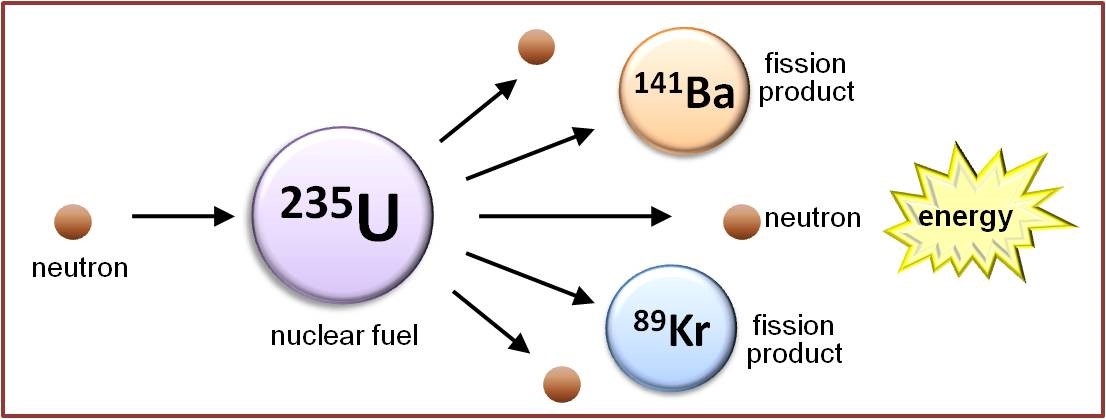
Nuclear Chain Reactions
Looking more closely at the figure above, it becomes evident that the nuclear fission of uranium-235 produces more neutrons (three in this example) than it consumes (one). This suggests the possibility that the neutrons released when one uranium-235 nucleus undergoes fission can induce the fission of up to three neighbouring uranium-235 nuclei, each of which will also release neutrons as it fissions, which can interact with other uranium-235 atoms, and so on, thus creating a self-sustaining nuclear chain reaction. It is this chain reaction that provides the basis for nuclear research reactors and nuclear power, as it releases not only neutrons and fission products, but also large quantities of energy.
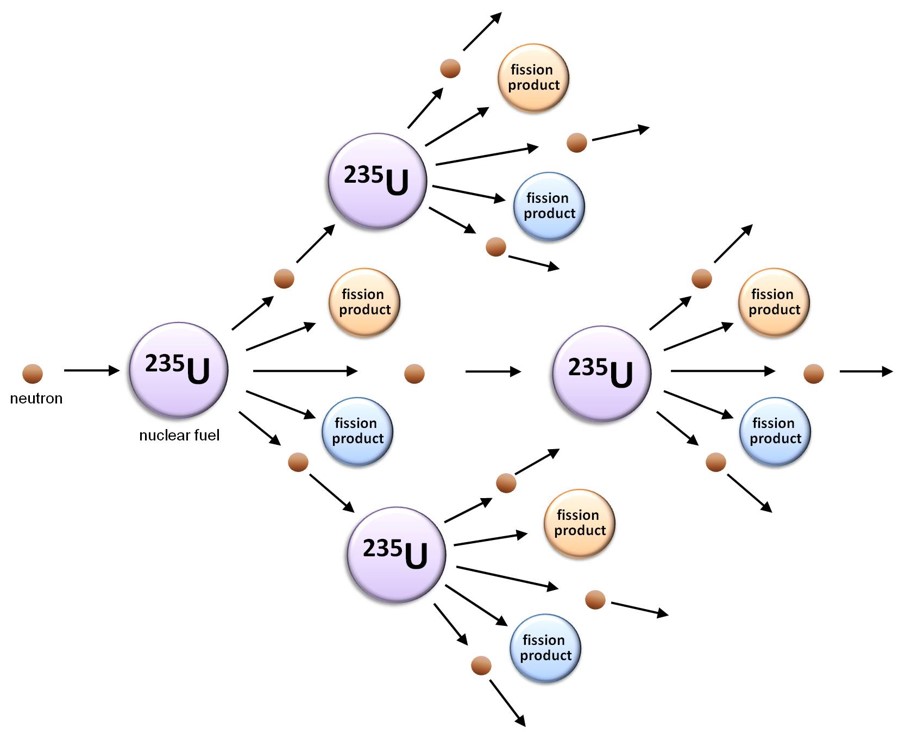
Controlling Fission
With a core of enriched uranium fuel assemblies in place, a nuclear reactor can be “started up” using a neutron source: a radioactive substance that emits enough neutrons to jump-start the nuclear chain reaction described above. One commonly used neutron source is californium-252, which spontaneously undergoes both alpha decay and nuclear fission, generating neutrons. Another option is to use a mixture of the chemical element beryllium and a radioactive isotope of antimony (antimony-124 or Sb-124): in this case, the high energy gamma ray given off by the Sb-124 as it undergoes radioactive decay interacts with the nearby beryllium atoms, causing them to eject neutrons. An antimony-124/beryllium neutron source encased in aluminum (so as to avoid contamination of the pool water) is used at MNR to initiate a nuclear chain reaction in the reactor core after protracted shutdowns.
Along with a means of instigating nuclear fission in the reactor core, it is important to be able to control the rate at which the uranium atoms are undergoing fission. If the nuclear chain reaction is allowed to propagate unchecked, the rate of fission will increase rapidly as more and more neutrons are produced, resulting in a massive and nearly instantaneous release of energy and heat. In contrast, in a nuclear reactor, the rate at which the nuclear fuel undergoes fission is rigorously controlled using “control rods”: cylinders of neutron absorbing material that are inserted into the reactor core to absorb a portion of the neutrons released by the fission of the uranium-235 fuel. To initiate a chain reaction when a reactor is being started up, the control rods are partially withdrawn so that they absorb fewer neutrons; once the reactor is critical (i.e. a chain reaction is occurring), the control rods are inserted slightly further into the core in order to slow the rate of fission to the required value and maintain it at that level.
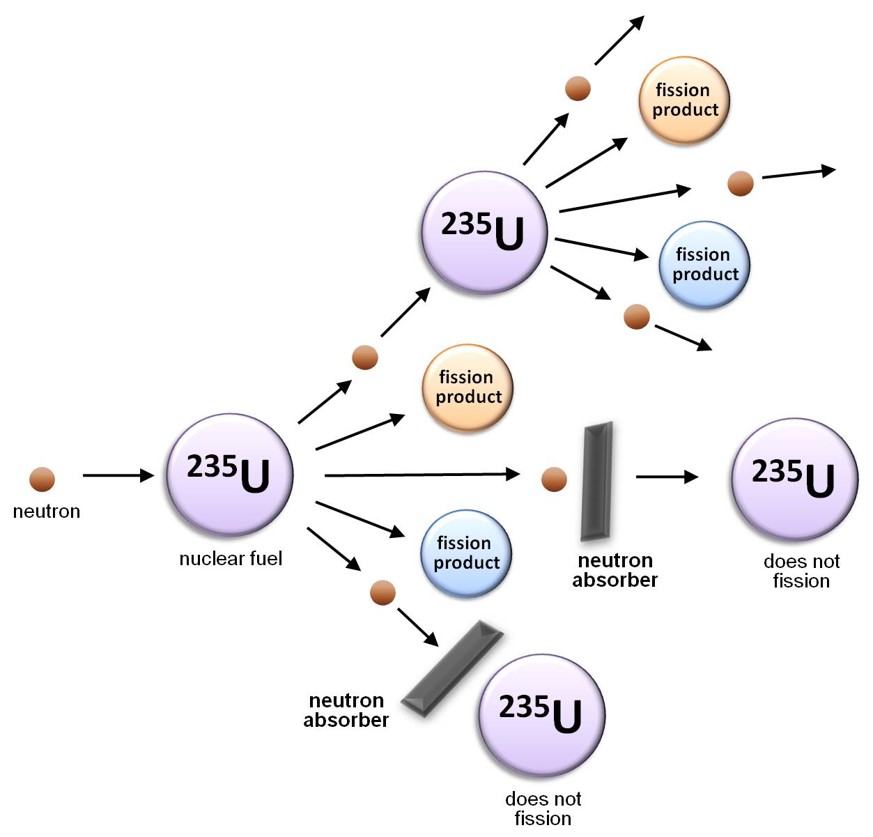
Control rods must be fabricated from a material that readily absorbs or “captures” neutrons, but is not prone to nuclear fission as a consequence. Materials appropriate for this application include boron carbide and the heavy metal cadmium. MNR uses control rods composed of an alloy of silver, indium and cadmium because each of these three metals is able to capture neutrons of a different kinetic energy than the other two, making the alloy highly efficient at regulating the neutron population or “flux” within the reactor core, and thereby controlling the rate of fission.

The other significant component in controlling the rate of fission in a nuclear reactor is the “moderator”. When uranium-235 undergoes fission, the atom flies apart, ejecting fission products and neutrons that are travelling at ~14,000 km/s (50 million km/h). These so-called fast neutrons can cause induced nuclear fission in other uranium-235 atoms, but the probability of this occurring is relatively low: neutron capture and subsequent fission is approximately a thousand times more likely to occur for slower-moving or “thermal” neutrons. The role of the neutron moderator is to decrease or moderate the speed (kinetic energy) of the fast neutrons and convert them to slow moving thermal neutrons to enhance nuclear fission. Of course, the term “slow” is relative: thermal neutrons are still travelling at approximately 2,200 m/s, or 7,920 km/h!
A material suitable for use as a moderator must be able to slow down neutrons without absorbing or “capturing” them. Commonly used neutron moderators include deionized (purified) water, “heavy water” (in which the hydrogen atoms of the water molecules are replaced by the heavier isotope deuterium) and graphite, a high-density form of carbon. Heavy water has the advantage of absorbing very few neutrons, allowing the nuclear chain reaction to progress efficiently, but is far more expensive than regular “light” water. Like the majority of nuclear reactors operational today, McMaster Nuclear Reactor utilizes stringently purified light water as its moderator, as the ready availability of light water is more than sufficient compensation for the decreased fission efficiency that results from its neutron absorptivity. In many nuclear reactors, including MNR, the moderator also doubles as a reactor coolant.
Nuclear Fuel
The key to initiating and maintaining a self-sustaining chain reaction that can serve as a source of either neutrons (for research) or energy (for power applications) is to select an appropriate fissile material, or “nuclear fuel”. The most common fuel is uranium-235 which undergoes nuclear fission readily when bombarded with slow-moving neutrons, as described above. Unfortunately, uranium-235 makes up only 0.72 % of natural uranium, the rest being uranium-238 and uranium-234: in consequence, the probability that the neutrons from the fission of a uranium-235 atom will bump in to another uranium-235 atom (and cause it to fission) is quite low. To overcome this stumbling block, the nuclear reactors that are operational today use uranium fuel that is enriched in uranium-235, with a U-235 concentration of 1-90 %. (The notable exceptions to this are the Canadian CANDU power reactors, which have a specialized design that allows them to sustain a nuclear chain reaction using natural uranium.) Regardless of the U-235 enrichment level, the uranium fuel “meat” in reactors is usually in the chemical form of uranium (IV) oxide, UO2, due to the high thermal stability and low chemical reactivity of this compound.
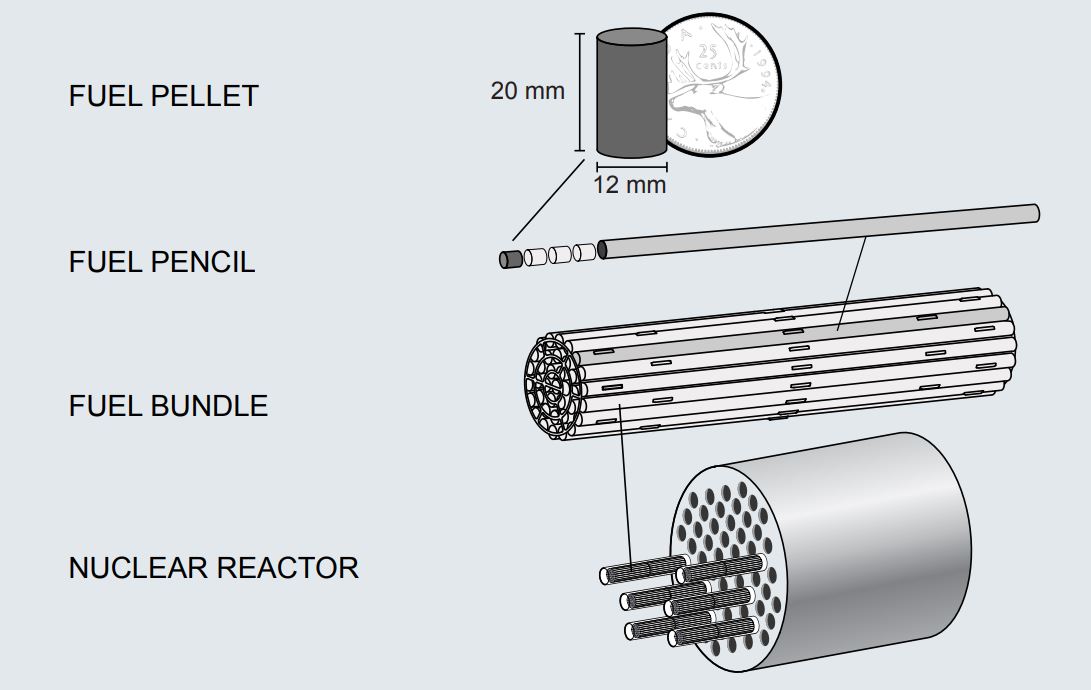
The McMaster Nuclear Reactor was originally designed to operate using Highly Enriched Uranium (HEU, >20 % U-235) fuel as were all reactors of its generation. In the 1990s, the global nuclear community shifted away from the use of HEU as a reactor fuel due to concerns that stockpiles of this material at civilian installations were too-convenient targets for malfeasant factions seeking to create nuclear weapons. At this time, MNR elected to participate in the Reduced Enrichment for Research and Test Reactors program that was coordinated by the US Department of Energy and the International Atomic Energy Agency (IAEA), working toward the goal of securing the global supply of HEU by curbing its use at research reactors. Over a 10-year period, MNR converted to Low Enrichment Uranium (LEU, <20 % U-235) nuclear fuel with a full LEU core in place in April 2007, bringing MNR in line with current international guidelines for the non-proliferation of nuclear materials.
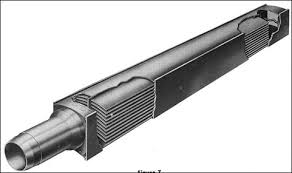

Rather than the familiar fuel “bundles” – actually numerous cylindrical pellets stacked end-to-end to form fuel “rods” that are grouped into “bundles” – that are used in many power reactors, MNR uses plate-style nuclear fuel. Each fuel plate consists of a mixture of sintered uranium silicide (U3Si2) and aluminum, with an outer coating (or “cladding”) of aluminum which prevents the uranium silicide and various fission products from dissolving into the reactor pool water and contaminating it. Fuel plates are stacked a fixed distance apart from one another inside an aluminum shell to form a “fuel assembly” (see image above). Several of these fuel assemblies are strategically arranged in the reactor pool to achieve a controlled, self-sustaining chain reaction, forming the core of the nuclear reactor.
Cooling System
Though this discussion has so far focused on the neutrons that are produced by nuclear fission, the fact that large quantities of energy are released must not be overlooked. This is reflected in the large kinetic energy that is imparted to the fast-moving neutrons and fission products as they dispersed from the fissioning atoms; energy is also released in the form of gamma radiation or gamma rays. As the fission products and fast neutrons travel through the neutron moderator and slow down, much of their kinetic energy is converted into thermal energy, or heat. Gamma rays are also attenuated by the moderator, resulting in the production of heat. In consequence, the core of a nuclear reactor needs to be cooled continuously in order to keep the structural elements of the core and the fuel assemblies from overheating or even melting.
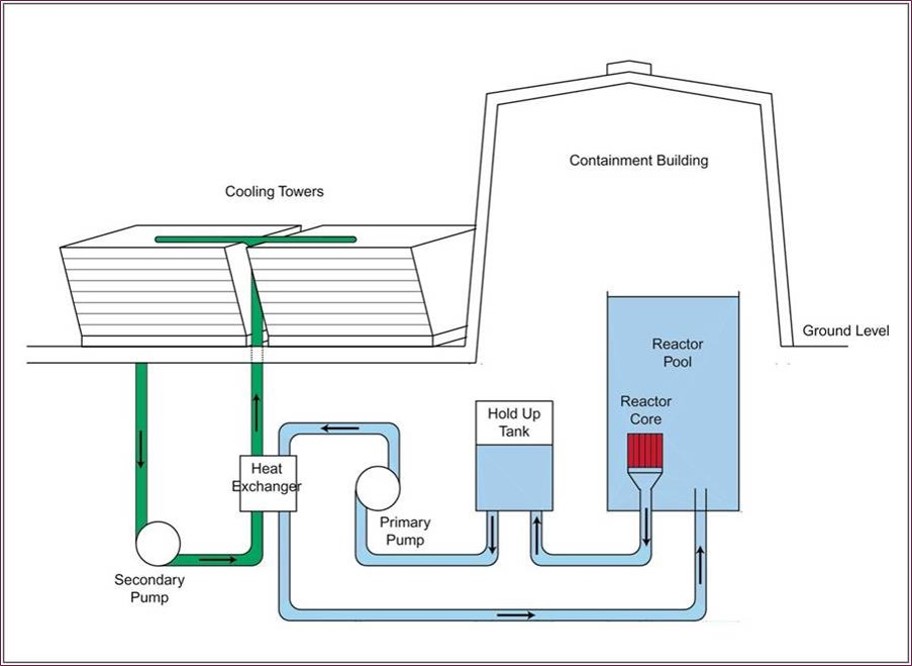
In many cases, the reactor’s neutron moderator also acts as a coolant. For example, at MNR, the reactor pool water (the moderator) absorbs the heat energy that is given off in the core. In order to prevent it from over-heating and boiling, the pool water is not stagnant: it circulates continuously downwards through the reactor core and out via a special aperture at the bottom of the pool, from where it is directed by the primary pump to a heat exchanger where its heat energy is transferred to the secondary cooling system before it is routed back into the reactor pool (see diagram above). The cooling towers (see images below) are designed to act as heat exchangers, removing heat (thermal energy) from the secondary cooling system (green) and transferring it to the atmosphere – the final destination for the energy created in the MNR core. Because the pool water (primary cooling system, light blue) does not come in direct contact with the cooling towers, any contaminants in the pool water cannot escape to the atmosphere. The cooling towers are located adjacent to the reactor containment building, wedged in between the Arthur Bournes Building and the Nuclear Research Building.

Structural Features
Reactor Design
Because MNR was constructed in the 1950s – a time when fossil fuels were inexpensive and concerns about environmental pollution were much less than they are today – there are no systems in place to recover and use the energy created at MNR. Moreover, the McMaster Nuclear Reactor was designed to be used for research purposes, not for power generation. With its end use in mind, an “open-pool” reactor design was implemented as it allows easy access to the reactor core for experimental purposes such as the insertion of samples for neutron irradiation.
While the majority of nuclear reactors that are intended for research purposes possess some variation on the open-pool design, nuclear reactors that are intended for energy production are quite different in appearance, though they function on the same operating principles. Rather than being immersed in a pool of moderator/coolant that is open to the atmosphere, the core of a power reactor is contained within a sealed vessel called a pressure vessel or calandria. The energy released by nuclear fission in the reactor core heats the (usually) light water moderator/coolant within the reactor vessel, and the hot coolant is circulated through a heat exchanger where the thermal energy (heat) of the coolant is used to generate steam. While the now-cooled coolant/moderator is recirculated into the reactor vessel, the steam is directed to a turbine that is connected to an electric generator: as the steam causes the turbine blades to turn, electricity is generated and routed into the local power grid.

The different design features of power generating reactors as opposed to research reactors is function of their differing end goals: while convenient access to the reactor core is the preferred characteristic of a research reactor, the focus in a power generating station is to efficiently capture the energy released by nuclear fission and convert it into useable (electrical) energy.
Containment Structures
Because nuclear power reactors generate large amounts of both energy and radioactivity, they are housed within leak-tight concrete “containment structures” that are designed to protect the surrounding area from exposure to radiation or radioactive materials. Each of these buildings – the distinctive concrete domes present at nuclear power plants – is engineered to withstand the maximum credible accident scenario for its particular reactor design and power, with the natural hazards of the region (e.g. earthquakes, floods, etc.) also taken into account. In the unlikely event that a severe accident scenario is realized, the containment structure will prevent nuclear material and radiation from being released into the atmosphere.

In contrast, nuclear research reactors are generally housed in buildings constructed of more standard materials such as wood or aluminum siding. This is due to the difference in scale between power reactors (several hundred megawatts of energy) and research reactors (less than ten megawatts): the much smaller research reactors do not require full concrete containment structures in order for the facility to be considered safe. When plans were initially made to have a nuclear reactor installed at McMaster, the facility was designed with maximum safety in mind, as the reactor was to be located in the middle of a densely populated university campus. The decision was made to include a full concrete containment structure for the MNR, though for a medium-flux (5 MW) reactor, a less robust structure would have been sufficient from a safety standpoint. The containment building at the McMaster Nuclear Reactor – a 15-sided concrete polyhedron – is one of the more recognizable structures on the McMaster University campus. Designed with maximum safety in mind, the foundation of the building is a 1.5 m thick reinforced concrete pad: the minor earthquakes that occur in the Hamilton region cannot be felt from within the containment building. The walls (70 cm thick concrete with reinforcing rods) and roof (minimum thickness of 30 cm) are of similarly sturdy construction.
